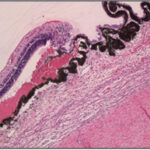
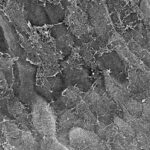
 Vitreoretinal adhesion is known to be an important factor in many ocular disorders as well as in traumatic eye injury. Detailed analyses of the structure of the vitreoretinal interface have been performed and it’s theorized that collagen fibers are the predominant source of adhesion. In some regions of the eye (vitreous base, posterior pole), the collagen fibers have been shown to traverse the vitreoretinal interface perpendicular to the surface of the retina. However, in other regions (equator), the collagen fibers run parallel to the surface and it’s thought that other collagen proteins lie between the vitreous cortex and retina acting as a type of ‘glue’ between the structures. This creates a regional variation in vitreoretinal adhesion. Furthermore, as an eye ages, the collagen breaks down. This would result in changes in vitreoretinal adhesion wiht age. To test this, we are currently quantifying collagen at the vitreoretinal interface and correlating to measurements of adhesion on a macroscopic and microscopic scale.
Vitreoretinal adhesion is known to be an important factor in many ocular disorders as well as in traumatic eye injury. Detailed analyses of the structure of the vitreoretinal interface have been performed and it’s theorized that collagen fibers are the predominant source of adhesion. In some regions of the eye (vitreous base, posterior pole), the collagen fibers have been shown to traverse the vitreoretinal interface perpendicular to the surface of the retina. However, in other regions (equator), the collagen fibers run parallel to the surface and it’s thought that other collagen proteins lie between the vitreous cortex and retina acting as a type of ‘glue’ between the structures. This creates a regional variation in vitreoretinal adhesion. Furthermore, as an eye ages, the collagen breaks down. This would result in changes in vitreoretinal adhesion wiht age. To test this, we are currently quantifying collagen at the vitreoretinal interface and correlating to measurements of adhesion on a macroscopic and microscopic scale.
Traumatic brain injury (TBI) is the most common cause of death in childhood. The predominant etiologies of TBI in young children are motor vehicle accidents, firearm incidents, falls, and child abuse. Unfortunately, falls are the leading cause of non-inflicted head injury in infants less than a year old, and are also the most common history provided by caretakers suspected of child abuse. The objective of this research proposal is to provide clinicians with a biomechanics-based tool to aide in the diagnosis of inflicted or non-inflicted trauma with a history of a low height fall.
Using dynamic material property data of infant cranial bone and suture, experimental studies of rapid head rotation, instrumented anthropomorphic surrogate simulations and finite element modeling, we plan to recreate several real-world cases of children < 1 year old experiencing a low height fall. By correlating the mechanical stresses and strains from these recreations with the injuries we can identify the best biomechanical parameters for predicting skull fracture and intracranial hemorrhage, and determine stress and strain thresholds for injury.
Supported by the Department of Justice
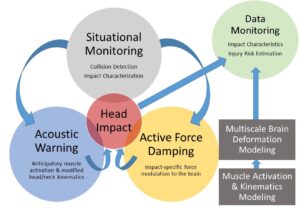
The goal of this proposal is to reduce the risk of traumatic brain injury (TBI) through smart technology that collects sensory data to predict and characterize impacts in real-time, optimizes protective mechanisms based on impact characteristics (e.g., direction, velocity), and transmits final impact attributes to a database for further analysis and injury risk prediction. This technology will substantially improve TBI prevention and diagnosis in motor vehicle crashes, sports, and industrial accidents. The unique technology will leverage musculoskeletal and biomechanical computational models linking head linear and angular acceleration to brain deformation and injury. To accomplish this goal, fundamental research efforts include (1) real-time situational monitoring to predict when and how dangerous impacts are about to occur and (2) active prevention mechanisms in the form of anticipatory muscle activation and event-specific force damping bladders to reduce the risk of TBI due to impact. Initial evaluation of the technology will occur in a sports setting, but the integrated system will be widely applicable to multiple etiologies of TBI.Supported by the National Science Foundation
 Finite element models are an important part of adult and pediatric vision research. Used correctly, they can significantly reduce the costs of vision research and accelerate the development of treatments for ocular abnormalities. They have been instrumental in adult ocular research, but finite element models for children are severely limited by the paucity of material properties available for the pediatric eye. Pediatric models currently use adult properties and assume there are no significant differences with age. Clinically, it is known that the mechanical response of ocular tissues in children is different than in adults, but these differences have never been quantified. One of the objectives in our research program is to develop a viable model of the pediatric eye that can be used by vision researchers to enhance and accelerate pediatric ocular research. This model would impact research on congenital glaucoma, strabismus, abusive head trauma, and many other pediatric disorders. However, before such a model can be developed, the material properties of pediatric ocular tissues must be characterized and correlated with age. Therefore, we propose a comprehensive quantification of the material properties of several pediatric ocular structures. Data will be collected from both human and animal resources to obtain a full understanding of the mechanical and structural changes that occur with age. Upon completion of this study, the data will be used to develop a finite element model of the pediatric eye that can spur new avenues of exploration for understanding and treating pediatric ocular disorders.
Finite element models are an important part of adult and pediatric vision research. Used correctly, they can significantly reduce the costs of vision research and accelerate the development of treatments for ocular abnormalities. They have been instrumental in adult ocular research, but finite element models for children are severely limited by the paucity of material properties available for the pediatric eye. Pediatric models currently use adult properties and assume there are no significant differences with age. Clinically, it is known that the mechanical response of ocular tissues in children is different than in adults, but these differences have never been quantified. One of the objectives in our research program is to develop a viable model of the pediatric eye that can be used by vision researchers to enhance and accelerate pediatric ocular research. This model would impact research on congenital glaucoma, strabismus, abusive head trauma, and many other pediatric disorders. However, before such a model can be developed, the material properties of pediatric ocular tissues must be characterized and correlated with age. Therefore, we propose a comprehensive quantification of the material properties of several pediatric ocular structures. Data will be collected from both human and animal resources to obtain a full understanding of the mechanical and structural changes that occur with age. Upon completion of this study, the data will be used to develop a finite element model of the pediatric eye that can spur new avenues of exploration for understanding and treating pediatric ocular disorders.
Supported by the Knights Templar Eye Foundation
 The objectives of this proposal are to characterize the progression of visual system injury from blast exposure, determine correlations between blast overpressure severity and progression of injury, and identify early predictors of vision degradation following blast exposure. To accomplish these goals we have developed a multidisciplinary approach that incorporates clinical and experimental studies. First we will identify the occurrence and time delay of visual system injury in military personnel from blast exposure. We will then use our findings to guide a longitudinal study tracking and monitoring the vision in military personnel. Additionally, we will evaluate early predictors for visual dysfunction identified from animal studies. Then, we will systematically examine the progression of visual system injury in our rodent blast injury model and determine early identifiers of visual system injury at different blast severities. Finally, we will look at proteomic changes associated with visual system injury and to identify if there are biomarkers indicative of vision degeneration. The successful completion of this project will provide significant insight into the progression of visual system injury from blast and lead to enhanced diagnostic capabilities and treatment options for military personnel.
The objectives of this proposal are to characterize the progression of visual system injury from blast exposure, determine correlations between blast overpressure severity and progression of injury, and identify early predictors of vision degradation following blast exposure. To accomplish these goals we have developed a multidisciplinary approach that incorporates clinical and experimental studies. First we will identify the occurrence and time delay of visual system injury in military personnel from blast exposure. We will then use our findings to guide a longitudinal study tracking and monitoring the vision in military personnel. Additionally, we will evaluate early predictors for visual dysfunction identified from animal studies. Then, we will systematically examine the progression of visual system injury in our rodent blast injury model and determine early identifiers of visual system injury at different blast severities. Finally, we will look at proteomic changes associated with visual system injury and to identify if there are biomarkers indicative of vision degeneration. The successful completion of this project will provide significant insight into the progression of visual system injury from blast and lead to enhanced diagnostic capabilities and treatment options for military personnel.
Supported by Department of Defense Vision Research Program
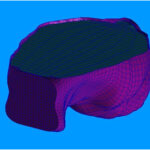 We hypothesize that children can sustain head injury from cyclic loading without impact to the skull. No data exists to test this theory. To date, we have designed a well-defined model of head trauma due to a single, rapid head rotation, but the device does not allow for repetitive loading. We have now designed and built a mechanical system capable of producing 3Hz of back and forth head rotation at acceleration loads similar to those measured in surrogate simulations of shaking. With this device we propose to measure the effect of a single cyclic head rotation versus cyclic head rotations on tissue injury, identify the effect of cyclic head injury on behavior and cognition, use finite element analysis to identify tissue strain from cyclic loading, and determine injury tolerances due to cyclic head rotation in children.
We hypothesize that children can sustain head injury from cyclic loading without impact to the skull. No data exists to test this theory. To date, we have designed a well-defined model of head trauma due to a single, rapid head rotation, but the device does not allow for repetitive loading. We have now designed and built a mechanical system capable of producing 3Hz of back and forth head rotation at acceleration loads similar to those measured in surrogate simulations of shaking. With this device we propose to measure the effect of a single cyclic head rotation versus cyclic head rotations on tissue injury, identify the effect of cyclic head injury on behavior and cognition, use finite element analysis to identify tissue strain from cyclic loading, and determine injury tolerances due to cyclic head rotation in children.
Supported by the Primary Children’s Medical Center Foundation